In the realm of biochemical processes, glycosylation and glycation stand as pivotal mechanisms with distinct roles and impacts on protein function. As essential processes in cellular biology, understanding their similarities and differences is crucial for unraveling their significance in health and disease. In this comprehensive article, we delve into the defining characteristics, biochemical processes, roles of sugars, impacts on proteins, and biological significance of glycosylation and glycation.
Defining Glycation and Glycosylation
Glycation:
Glycation is a non-enzymatic process wherein free sugars, such as glucose, fructose, or galactose, covalently bind to proteins. This spontaneous reaction occurs in the bloodstream, leading to the formation of AGEs. Glycation is associated with protein damage, reducing stability and functionality.
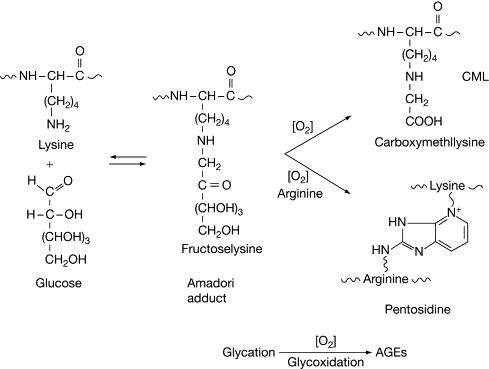 Pathway for glycation of protein. (M. Lima, et al, Encyclopedia of Biological Chemistry (Second Edition), 2013)
Pathway for glycation of protein. (M. Lima, et al, Encyclopedia of Biological Chemistry (Second Edition), 2013)
Select Service
Glycosylation:
Glycosylation, in contrast, is an enzymatic process facilitated by glycosyltransferases. It involves the attachment of predefined sugar molecules, like glucose, mannose, or glycans, to specific amino acid residues on proteins. Glycosylation occurs in the endoplasmic reticulum and Golgi apparatus and plays a crucial role in protein folding, stability, and functionality.
The Biochemical Process of Glycation
Condensation and Formation of Schiff Base
The introductory phase of glycation commences with the coupling of a reducing sugar, potentially glucose, fructose, or galactose, with the protein's amino group. This process is a non-enzymatic reaction that effortlessly unfolds under physiological circumstances. The anticipation of the initial stage involves the formation of a reversible Schiff base or an aldimine through a key interaction between the carbonyl group of the sugar molecule and the amino group within the protein structure. It is noteworthy to reference the ground-breaking work of Brownlee et al. (1986), in which they explicated the condensation reaction occurring between glucose and protein amino groups. Their study delineated the successive creation of Schiff bases, instrumental as intermediaries during the glycation progression.
Rearrangement to Amadori Product
Following the establishment of the Schiff base, an intramolecular rearrangement ensues, culminating in the conversion of the transient Schiff base to a more enduring Amadori product. This transformative process involves the migration of the protonated amino group within the sugar moiety, facilitating the establishment of a stable ketoamine linkage between the sugar and the protein. Notably, Thornalley (1990) provided empirical evidence elucidating the in vitro conversion of Schiff bases to Amadori products, thereby underscoring the pivotal role of this rearrangement in the trajectory of glycation reactions.
Formation of AGEs
As time progresses, Amadori products undergo successive chemical modifications, including oxidative processes, dehydration phenomena, and rearrangement events, ultimately leading to the formation of AGEs. These irreversible alterations precipitate the accrual of highly heterogeneous protein adducts characterized by modified structural and functional attributes. For instance, Sell et al. (1992) meticulously characterized the diverse chemical compositions of AGEs originating from diverse protein-sugar interactions, thereby spotlighting the intricate nature of glycation-derived modifications within biological milieus.
Biological Consequences of Glycation
The accrual of AGEs within tissues and organs elicits profound biological ramifications, substantially fueling the pathogenesis of a myriad of age-related afflictions, encompassing diabetes, cardiovascular maladies, and neurodegenerative conditions. AGEs notably engage with specific receptors, prominently the receptor for advanced glycation end products (RAGE), thereby inciting the activation of inflammatory cascades and oxidative stress pathways. Exemplifying this intricate interplay, Bierhaus et al. (2005) conducted seminal research elucidating the pivotal role of AGE-RAGE interactions in exacerbating vascular dysfunction and precipitating atherosclerotic processes, thereby accentuating the pathological implications of glycation in cardiovascular morbidities.
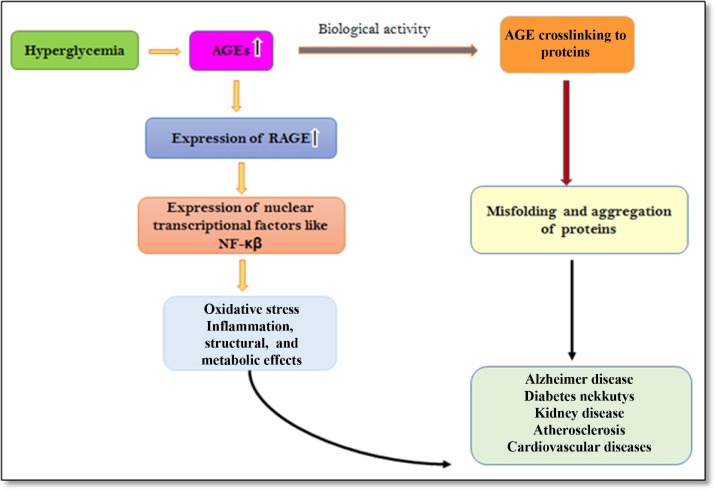 Mechanism of action of advanced glycation end products (AGEs). (PrairnaBalyan ,et al, Black Seeds (Nigella Sativa), 2022)
Mechanism of action of advanced glycation end products (AGEs). (PrairnaBalyan ,et al, Black Seeds (Nigella Sativa), 2022)
The Biochemical Process of Glycosylation
N-Linked Glycosylation
N-linked glycosylation stands as a prevalent post-translational modification, orchestrated within the confines of the endoplasmic reticulum and Golgi apparatus. In this meticulously orchestrated process, oligosaccharide chains become covalently affixed to the nitrogen atom of asparagine residues nestled within the consensus sequence Asn-X-Ser/Thr (where X denotes any amino acid barring proline). Facilitating this modification is the enzyme oligosaccharyltransferase, tasked with the transfer of the oligosaccharide precursor from a lipid carrier onto the emergent polypeptide chain.
A pivotal contribution by Helenius and Aebi (2001) yielded a comprehensive exposition on N-linked glycosylation pathways alongside their regulatory machinery, thereby unraveling the intricate symbiosis between glycan biosynthesis and protein folding within the secretory itinerary.
O-Linked Glycosylation
O-linked glycosylation encompasses the attachment of glycan moieties to the hydroxyl group of serine or threonine residues nestled within proteins. Diverging from N-linked glycosylation, which transpires co-translationally within the precincts of the endoplasmic reticulum, O-linked glycosylation predominantly unfolds post-translationally within the Golgi apparatus. This intricate process unfolds under the catalytic guidance of various glycosyltransferases, orchestrating the sequential addition of monosaccharides onto designated serine or threonine residues, thus yielding an array of structurally diverse O-glycan configurations.
A seminal investigation by Hart and Copeland (2010) underscored the intricacy inherent in O-linked glycosylation pathways alongside the multifaceted functional repertoire wielded by O-glycan modifications in modulating protein stability, subcellular localization, and molecular interactions.
Role of Glycosyltransferases
Glycosyltransferases assume a central role in orchestrating glycosylation processes by effectuating the transfer of sugar residues from activated nucleotide sugar donors onto designated acceptor substrates. These enzymatic entities evince discernible substrate preferences and undergo regulatory modulation across various tiers, encompassing gene expression modulation, subcellular localization dynamics, and post-translational adjustments.
Illustratively, the investigative endeavors of Brockhausen et al. (2009) delineated the substrate-specific nuances and catalytic intricacies inherent in glycosyltransferases engaged in the synthesis of intricate glycan architectures. This scholarly pursuit illuminated the underpinnings of glycosylation's molecular diversity and intricacy, thus enriching our comprehension of this biological phenomenon.
Glycosylation in Protein Quality Control
Glycosylation emerges as a pivotal player in the intricate landscape of protein quality control mechanisms, exerting regulatory influence over protein folding dynamics, stability profiles, and degradation processes. The fidelity of glycosylation is paramount in ensuring the accurate folding and intracellular trafficking of proteins. Disruptions in glycosylation patterns can precipitate protein misfolding events and the aggregation phenomena, thereby exacerbating the pathogenesis of protein folding disorders, including Alzheimer's disease and cystic fibrosis.
For instance, a seminal inquiry by Helenius and Aebi (2004) provided compelling evidence elucidating the indispensable role of glycosylation in orchestrating protein quality control pathways. This investigative endeavor underscored the critical significance of glycan-mediated mechanisms in upholding cellular equilibrium and proteostatic balance.
Similarities Between Glycation and Glycosylation
Covalent Modification of Proteins
A fundamental convergence between glycation and glycosylation lies in their shared capacity to effectuate covalent modifications upon proteins via the addition of sugar moieties. In both instances, sugars are affixed onto designated amino acid residues within protein entities, thereby instigating alterations in their chemical attributes and functional properties. Such covalent alterations predominantly transpire through nucleophilic addition reactions, wherein reactive functional groups on sugar molecules engage with amino acid side chains within the protein framework.
Exemplifying this chemical interplay, a comprehensive investigation conducted by Varki et al. (2015) elucidated the underlying chemical principles governing protein glycosylation and glycation phenomena. This scholarly pursuit underscored the pivotal role of nucleophilic catalysis alongside stereochemical considerations in dictating the specificity and efficacy of these transformative processes.
Impact on Protein Structure and Function
Both glycation and glycosylation exert significant influence on the structural integrity, stability, and functionality of proteins. Through the addition of sugar molecules, these processes introduce structural heterogeneity and chemical diversity into protein entities, thereby modulating their biochemical attributes and biological functionalities.
Illustratively, glycosylation emerges as a potent mechanism capable of enhancing protein stability, solubility, and resilience against proteolytic degradation, thereby facilitating the intricate processes of protein folding and assembly. Conversely, glycation poses a threat to protein functionality by promoting aggregation phenomena, perturbing enzyme activities, and disrupting crucial molecular interactions.
The scholarly endeavors of Spiro (2002) provided invaluable insights into the structural ramifications arising from protein glycosylation and glycation, thereby elucidating their profound impact on protein conformational dynamics, ligand-binding modalities, and the intricate landscape of cellular signaling pathways.
Regulatory Roles in Cellular Processes
Both the process of glycosylation and glycation are implicated in the regulation of a multitude of cellular procedures, encompassing cell signal propagation, immune responses, as well as the orchestration of developmental pathways. Modifications mediated via glycosylation have the potential to influence interactions between proteins, recognition between cells, as well as ligand-receptor binding events. Subsequently, these alterations can steer both intracellular signaling pathways and processes involved in extracellular communication. Analogously, modifications instigated through glycation might wield an impact on both protein turnover and intracellular localization, as well as modifying enzymatic operations. These alterations can, in turn, influence cellular equilibrium and the flux of metabolic processes. Scholarly investigations led by Varki in 2017 and a collective study spearheaded by Varki et al. in 2009 illuminated the diverse functionalities attributable to both glycosylation and glycation in cellular physiology, along with pathophysiology. Such findings underscore the importance of both mechanisms as instrumental regulators operating within both the realm of health and disease.
Pathophysiological Implications
Both glycation and glycosylation bear significant pathophysiological ramifications across a spectrum of diseases, prominently including diabetes, neurodegenerative disorders, and cancer. Dysregulated glycation processes, typified by the accumulation of AGEs, prominently contribute to the genesis and progression of diabetic complications, encompassing retinopathy, nephropathy, and neuropathy. Correspondingly, anomalous glycosylation patterns correlate with cancer metastasis, immune evasion mechanisms, and inflammatory responses within tumor microenvironments.
Pioneering investigations by Thornalley (2005) and Hart et al. (2011) have yielded comprehensive insights into the pathophysiological sequelae arising from glycation and glycosylation dysregulation. These seminal inquiries underscore the pivotal roles of these processes as discernible diagnostic biomarkers and promising therapeutic targets within the ambit of disease management and intervention strategies.
Difference between Glycosylation and Glycation
Enzymatic vs. Non-Enzymatic Processes
One of the fundamental distinctions between glycosylation and glycation lies in the underlying biochemical mechanisms. Glycosylation represents an enzymatic post-translational modification predominantly occurring within the endoplasmic reticulum and Golgi apparatus, orchestrated by specific glycosyltransferase enzymes. These enzymes facilitate the precise and regulated addition of sugar moieties, such as glucose, mannose, or galactose, to targeted proteins. In contrast, glycation manifests as a non-enzymatic process, spontaneously unfolding within the bloodstream, devoid of enzymatic mediation. It entails the indiscriminate attachment of free reducing sugars, such as glucose, fructose, or galactose, to protein amino groups via Maillard reactions.
For instance, research endeavors by Varki et al. (2017) have provided elucidations into the enzymatic machinery and regulatory frameworks governing protein glycosylation. Their work accentuated the pivotal role of glycosyltransferase enzymes in orchestrating site-specific glycan attachment to protein substrates. Conversely, investigations conducted by Thornalley (2005) and Brownlee (2001) have shed light on the non-enzymatic pathways of protein glycation. Their studies underscored the significance of reactive carbonyl compounds and sugar derivatives in instigating glycation reactions across diverse physiological and pathological contexts.
Site-Specificity and Regulation
An additional critical disparity between glycosylation and glycation pertains to the extent of site-specificity and regulatory oversight characterizing these processes. Glycosylation reactions demonstrate remarkable specificity, honing in on precise amino acid residues within protein sequences for glycan attachment. This specificity is driven by consensus recognition motifs and the substrate specificity of glycosyltransferases. Such site-specificity ensures the exact modification of proteins with well-defined glycan structures, thereby facilitating a myriad of biological functions and molecular interactions. In contrast, glycation reactions lack such specificity and unfold randomly, resulting in the indiscriminate modification of protein amino groups scattered across the polypeptide chain.
For instance, investigations by Helenius and Aebi (2004) delved into the intricate mechanisms governing the site-specificity of N-linked glycosylation within the endoplasmic reticulum. Their work underscored the pivotal roles played by glycosylation sequons and protein folding dynamics in directing glycan attachment to specific asparagine residues. Conversely, studies by McLellan et al. (1994) and Kilhovd et al. (1999) unveiled the non-specific nature of protein glycation reactions. Their findings revealed widespread modifications of lysine and arginine residues by reducing sugars, observed in diabetic patients and aging individuals, highlighting the indiscriminate nature of glycation processes.
Functional Consequences
The distinctions in enzymatic regulation and site-specificity between glycosylation and glycation precipitate discernible functional outcomes for the proteins undergoing modification. Glycosylation typically engenders the generation of structurally diverse glycan appendages affixed to proteins. These glycan entities wield the capacity to modulate various aspects of protein biology, including folding dynamics, stability profiles, solubility characteristics, and intracellular trafficking pathways. Such glycan modifications serve indispensable functions in protein quality control mechanisms, immune recognition processes, cellular adhesion events, and signal transduction cascades.
In contrast, modifications induced by glycation predominantly culminate in the formation of AGEs. These AGEs manifest as highly stable aggregates of cross-linked proteins, characterized by altered biochemical properties. For instance, investigations conducted by Varki et al. (2009) and Dwek (1996) underscored the functional significance of protein glycosylation across a spectrum of biological phenomena, encompassing cell-cell interactions, host-pathogen recognition, and antibody-mediated immune responses. Conversely, studies by Monnier et al. (1996) and Singh et al. (2001) elucidated the deleterious effects arising from protein glycation and AGE accrual in diabetic complications, neurodegenerative ailments, and cardiovascular disorders. These findings underscore the pivotal role of glycation in precipitating protein malfunction and tissue pathology.
Summary – Glycation vs Glycosylation
| Aspect | Glycation | Glycosylation |
|---|---|---|
| Definition | Glycation is the non-enzymatic process of covalently adding free sugars, such as glucose, fructose, or galactose, to proteins, occurring spontaneously in the bloodstream. | Glycosylation is a post-translational modification process occurring in the endoplasmic reticulum and Golgi apparatus, where sugars like xylose, fucose, mannose, or glycans are enzymatically added to proteins to produce functional proteins. |
| Regulation | Glycation is not regulated by enzymes, leading to the non-specific addition of sugars to proteins, thereby reducing their stability and functionality. | Glycosylation, in contrast, is an enzyme-regulated process that precisely adds sugars to specific regions of proteins, ensuring proper protein function and stability. |
| Significance | Glycation adds sugars randomly to proteins, resulting in the formation of non-functional proteins. | Glycosylation, on the other hand, adds sugars in a controlled manner, leading to the production of functional proteins essential for various biological processes. |
| Enzyme Involvement | Glycation does not involve enzymes; it is a spontaneous chemical reaction between sugars and proteins. | Glycosylation requires specific enzymes that catalyze the addition of sugars to proteins at specific sites, ensuring proper protein structure and function. |
| Effect on Protein Functionality | Glycation reduces protein functionality by altering its structure and impairing its normal function. | Glycosylation enhances protein functionality by facilitating proper folding and post-translational modification, ultimately contributing to the protein's biological activity. |
| Effect on Protein Stability | Glycation decreases protein stability, making the protein more susceptible to degradation and dysfunction. | Glycosylation increases protein stability by promoting proper folding and preventing misfolding, thereby extending the protein's lifespan and functionality. |
The Function of Glycation and Glycosylation
The Role of Sugars in Glycation and Glycosylation
Sugars assume pivotal roles in both glycation and glycosylation processes, albeit yielding distinct outcomes. Within glycation, reducing sugars like glucose, fructose, and galactose engage with proteins, culminating in the formation of AGEs. Notably, research by Vlassara et al. (2002) has underscored the repercussions of glucose-induced glycation, elucidating its implications in diabetic complications such as nephropathy and retinopathy.
In stark contrast, glycosylation entails the enzymatic incorporation of predetermined sugar molecules onto proteins, thereby ensuring requisite protein folding dynamics and functional integrity. This enzymatic process holds paramount importance in the synthesis of glycoproteins pivotal for diverse cellular processes. For instance, investigations by Freeze et al. (2019) have elucidated the indispensable role of glycosylation in synthesizing glycoproteins critical for facilitating cell-cell recognition events and modulating signaling pathways.
The Impact of Glycation and Glycosylation on Proteins
The phenomena of glycation and glycosylation exert distinct yet consequential effects as they pertain to the stability and functionality of proteins. The former, glycation, is characterized by the non-enzymatic conjugation of sugar molecules with proteins, a process which can culminate in protein degradation and a subsequent diminishment of stability. Groundbreaking research conducted by Ahmed and colleagues in 2005 evidenced that such modifications to proteins via glycation debilitate enzyme activity and disrupt the seamless operability of cellular functions. These detrimental changes have been implicated as contributory factors in the pathogenesis of myriad diseases, including metabolic dysregulations such as diabetes and neurodegenerative disorders.
Conversely, the process of glycosylation augments protein stability and functionality, fostering correct protein folding and timely post-translational modification. This enzymatically controlled process governs the structure and function of proteins, thereby enabling cellular processes to operate seamlessly. Research spearheaded by Helenius and Aebi in 2004 underscored the pivotal role of glycosylation in the orchestration of protein folding and stringent quality control mechanisms in the endoplasmic reticulum. These cellular operations are critical for maintaining cellular homeostasis and optimal function.
The Biological Significance of Glycation and Glycosylation
Both glycation and glycosylation serve as cornerstone processes in cellular physiology and pathology, albeit operating through disparate mechanisms. Glycosylation stands as a pivotal mechanism for the synthesis of functional glycoproteins integral to cell adhesion, signaling cascades, and immune responses. Perturbations in glycosylation pathways are intricately linked to an array of diseases, encompassing congenital disorders of glycosylation (CDG) and cancer. Seminal research by Freeze and Stanley (2013) has illuminated the clinical ramifications of glycosylation aberrations in CDG, thereby accentuating the profound impact of glycosylation on human health and disease.
Conversely, dysregulation of glycation pathways precipitates the accrual of AGEs, contributing significantly to the pathogenesis of chronic ailments such as diabetes, cardiovascular diseases, and neurodegenerative disorders. Investigations by Singh et al. (2014) have underscored the pathological sequelae stemming from AGE formation in diabetes-related complications, thereby underscoring the imperative for therapeutic modalities targeting glycation pathways.
In summary, glycation and glycosylation represent indispensable biochemical processes, each wielding distinct roles in cellular function and disease etiology. A comprehensive comprehension of their functionalities and impacts on protein dynamics stands as a prerequisite for elucidating disease mechanisms and formulating targeted therapeutic interventions.
Conclution
In essence, glycation and glycosylation represent fundamental biochemical pathways orchestrating the modification of proteins through sugar moieties. While glycation unfolds as a non-enzymatic cascade culminating in the production of advanced glycation end products and protein impairment, glycosylation emerges as an enzymatically regulated mechanism indispensable for orchestrating proper protein folding, stability, and functionality. Despite their disparate modalities, both glycation and glycosylation exert indispensable roles in cellular biology, wielding profound implications for the panorama of health and disease.
As a pioneering entity in the realm of biotechnology, Creative Proteomics steadfastly maintains its position at the vanguard of scientific inquiry, diligently unraveling the intricate tapestry of glycation and glycosylation. Our commitment remains unwaveringly devoted to fostering innovative therapeutic paradigms aimed at modulating these pivotal processes, thus advancing the frontiers of medical intervention and fostering enhanced health outcomes.
References
- Brownlee, M., Cerami, A., Vlassara, H. (1988). Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. New England Journal of Medicine, 318(20), 1315-1321.
- Helenius, A., Aebi, M. (2001). Intracellular functions of N-linked glycans. Science, 291(5512), 2364-2369.
- Thornalley, P. J. (1990). The glyoxalase system in health and disease. Molecular aspects of medicine, 11(6), 317-343.
- Sell, D. R., Monnier, V. M., Vlassara, H. (1992). Aging, diabetes, and renal failure catalyze the oxidation of lysyl residues to 2-amino-6-oxo-1, 6-dihydropyrimidine. Diabetes, 41(6), 809-814.